Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Tanaka, Kazuya; Yamaji, Keiko*; Masuya, Hayato*; Tomita, Jumpei; Ozawa, Mayumi*; Yamasaki, Shinya*; Tokunaga, Kohei; Fukuyama, Kenjin*; Ohara, Yoshiyuki*; Maamoun, I.*; et al.
Chemosphere, 355, p.141837_1 - 141837_11, 2024/05
In this study, biogenic Mn(IV) oxide was applied to remove Ra from mine water collected from a U mill tailings pond in the Ningyo-toge center. Just 7.6 mg of biogenic Mn(IV) oxide removed more than 98% of the  Ra from 3 L of mine water, corresponding to a distribution coefficient of 10
Ra from 3 L of mine water, corresponding to a distribution coefficient of 10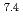 mL/g for Ra at pH 7. The obtained value was convincingly high for practical application of biogenic Mn(IV) oxide in water treatment.
mL/g for Ra at pH 7. The obtained value was convincingly high for practical application of biogenic Mn(IV) oxide in water treatment.
Wang, Y. W.*; Wang, H. H.*; Su, Y. H.; Xu, P. G.; Shinohara, Takenao
Materials Science & Engineering A, 887, p.145768_1 - 145768_13, 2023/11
Times Cited Count:1 Percentile:44.33(Nanoscience & Nanotechnology) -type layered manganese dioxide
-type layered manganese dioxideOkamoto, Norihiko*; Yoshisako, Hiroki*; Ichitsubo, Tetsu
Energy Storage Materials, 61, p.102912_1 - 102912_9, 2023/08
Times Cited Count:2 Percentile:62.48(Chemistry, Physical)Kozawa, Tatsuya*; Fujihara, Masayoshi; Uchihara, Takeru*; Mitsuda, Setsuo*; Yano, Shinichiro*; Tamatsukuri, Hiromu; Munakata, Koji*; Nakao, Akiko*
Scientific Reports (Internet), 13, p.13750_1 - 13750_8, 2023/08
Times Cited Count:0 Percentile:0(Multidisciplinary Sciences)In condensed matter physics, pressure is frequently used to modify the stability of both electronic states and atomic arrangements. Under isotropic pressure, the intermetallic compound MnP has recently attracted attention for the interplay between pressure-induced superconductivity and complicated magnetic order in the vicinity. By contrast, we use uniaxial stress, a directional type of pressure, to investigate the effect on the magnetism and crystal structure of this compound. An irreversible magnetisation response induced by uniaxial stress is discovered in MnP at uniaxial stress as low as 0.04 GPa. Neutron diffraction experiments reveal that uniaxial stress forms crystal domains that satisfy pseudo-rotational symmetry unique to the MnP-type structure. The structure of the coexisting domains accounts for the stress-induced magnetism. We term this first discovered phenomenon atomic reconstruction (AR) induced by uniaxial stress. Furthermore, our calculation results provide guidelines on the search for AR candidates. AR allows crystal domain engineering to control anisotropic properties of materials, including dielectricity, elasticity, electrical conduction, magnetism and superconductivity. A wide-ranging exploration of potential AR candidates would ensure that crystal domain engineering yields unconventional methods to design functional multi-domain materials for a wide variety of purposes.
Shiraishi, Fumito*; Chihara, Ryoji*; Tanimoto, Risa*; Tanaka, Kazuya; Takahashi, Yoshio*
Island Arc, 31(1), p.e12448_1 - e12448_9, 2022/05
Times Cited Count:0 Percentile:0(Geosciences, Multidisciplinary)At Yunotaki Fall in north Japan, manganese-oxidizing bacteria were previously assumed to have oxidized manganese to precipitate birnessite, which relied on oxygen released from algae. However, it remained unclear whether larger-scale manganese oxide precipitation was actually occurring under light conditions. This study evaluated the contribution of indirect oxidation using microelectrodes to analyze local water chemistry, in addition to bulk water chemistry and DNA analyses. The results of this study demonstrate that low bulk pH values in the hot spring water hindered indirect oxidation despite the occurrence of active oxygenic photosynthesis and that direct oxidation by manganese-oxidizing bacteria is considered to dominate in the investigated sample.
 and Mn
and Mn by fungal manganese oxide through asbolane formation
by fungal manganese oxide through asbolane formationAoshima, Miku*; Tani, Yukinori*; Fujita, Rina*; Tanaka, Kazuya; Miyata, Naoyuki*; Umezawa, Kazuhiro*
Minerals (Internet), 12(3), p.358_1 - 358_16, 2022/03
Times Cited Count:5 Percentile:72.8(Geochemistry & Geophysics)In this study, we conducted sequestration experiments of Co by
by 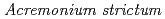 KR21-2 in a Mn
KR21-2 in a Mn /Co
/Co binary solution at pH7. The sequestration of Co
binary solution at pH7. The sequestration of Co by newly formed BMOs readily progressed in parallel with the exogenous Mn
by newly formed BMOs readily progressed in parallel with the exogenous Mn , with higher efficiency than that in single Co
, with higher efficiency than that in single Co solutions. This is attributed to a synergetic effect on Co
solutions. This is attributed to a synergetic effect on Co sequestration through the formation of asbolane in Mn
sequestration through the formation of asbolane in Mn /Co
/Co binary systems.
binary systems.
Tanaka, Kazuya; Tani, Yukinori*; Kozai, Naofumi; Onuki, Toshihiko
Journal of Radioanalytical and Nuclear Chemistry, 331(2), p.1109 - 1114, 2022/02
Times Cited Count:0 Percentile:0.01(Chemistry, Analytical)We investigated the sorption of Pu(IV) on biogenic Mn oxide produced by Mn(II)-oxidizing fungus. The sorption of Pu(IV) on biogenic Mn oxide was similar to that of U(VI) and different from that of Th(IV), possibly due to oxidation of Pu(IV) to Pu(VI). When Pu(IV) was sorbed on hyphae only, it was desorbed into the solution phase over time. Pu(IV) could be solubilized by complexation with organic ligands secreted by fungal cells. In particular, Pu(IV) desorption was observed under circumneutral pH conditions.
 through irreversible biogenic manganese oxide sequestration
through irreversible biogenic manganese oxide sequestrationTani, Yukinori*; Kakinuma, Satomi*; Chang, J.*; Tanaka, Kazuya; Miyata, Naoyuki*
Minerals (Internet), 11(1), p.53_1 - 53_14, 2021/01
Times Cited Count:4 Percentile:41.7(Geochemistry & Geophysics)In this study, we examined the Ba sequestration potential of enzymatically active biogenic Mn oxides (BMOs) with and without exogenous Mn
sequestration potential of enzymatically active biogenic Mn oxides (BMOs) with and without exogenous Mn . The BMOs readily oxidized exogenous Mn
. The BMOs readily oxidized exogenous Mn to produce another BMO phase, and subsequently sequestered Ba
to produce another BMO phase, and subsequently sequestered Ba at a pH of 7.0, with irreversible Ba
at a pH of 7.0, with irreversible Ba sequestration as the dominant pathway. Extended X-ray absorption fine structure spectroscopy and X-ray diffraction analyses demonstrated alteration from turbostratic to tightly stacked birnessite through possible Ba
sequestration as the dominant pathway. Extended X-ray absorption fine structure spectroscopy and X-ray diffraction analyses demonstrated alteration from turbostratic to tightly stacked birnessite through possible Ba incorporation into the interlayer.
incorporation into the interlayer.
Suzuki, Ryohei*; Tani, Yukinori*; Naito, Hirotaka*; Miyata, Naoyuki*; Tanaka, Kazuya
Catalysts, 10(1), p.44_1 - 44_15, 2020/01
Times Cited Count:10 Percentile:38.83(Chemistry, Physical)We prepared biogenic Mn oxides (BMOs) using 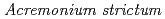 strain KR21-2, and subsequently conducted single or repeated treatment experiments in Cr(NO
strain KR21-2, and subsequently conducted single or repeated treatment experiments in Cr(NO )
) at pH6.0. Under aerobic conditions, newly formed BMOs exhibited a rapid production of Cr(VI) without a significant release of Mn(II), demonstrating that newly formed BMO mediates a catalytic oxidation of Cr(III) with a self-regeneration step of reduced Mn. In anaerobic solution, newly formed BMOs showed a cessation of Cr(III) oxidation in the early stage of the reaction, and subsequently had a much smaller Cr(VI) production with significant release of reduced Mn(II).
at pH6.0. Under aerobic conditions, newly formed BMOs exhibited a rapid production of Cr(VI) without a significant release of Mn(II), demonstrating that newly formed BMO mediates a catalytic oxidation of Cr(III) with a self-regeneration step of reduced Mn. In anaerobic solution, newly formed BMOs showed a cessation of Cr(III) oxidation in the early stage of the reaction, and subsequently had a much smaller Cr(VI) production with significant release of reduced Mn(II).
 , Zn
, Zn +, and Co
+, and Co
Kato, Tomoaki*; Yu, Q.*; Tanaka, Kazuya; Kozai, Naofumi; Saito, Takumi*; Onuki, Toshihiko
Journal of Environmental Sciences, 86, p.78 - 86, 2019/12
Times Cited Count:2 Percentile:8.12(Environmental Sciences)This paper investigated the fate of the dissolved permanganate in aqueous solution after contact with bacterial cells and metal accumulation during precipitation of Mn oxides. When Mn(VII) was contacted with bacterial cells, cells were damaged and Mn(VII) was reduced by cells to lower valence and precipitated as Mn oxides (biomass Mn oxides). When Co ions were present, Co was incorporated into Mn oxides as Co
ions were present, Co was incorporated into Mn oxides as Co . These results suggest that Mn(VII) can be used to remove metal ions when introduced to wastewater as disinfectant.
. These results suggest that Mn(VII) can be used to remove metal ions when introduced to wastewater as disinfectant.
Yu, Q.*; Tanaka, Kazuya; Kozai, Naofumi; Sakamoto, Fuminori; Tani, Yukinori*; Onuki, Toshihiko
ACS Earth and Space Chemistry (Internet), 2(8), p.797 - 810, 2018/08
Times Cited Count:14 Percentile:57.45(Chemistry, Multidisciplinary)Most of Mn oxides are biogenic and known to adsorb cesium (Cs) on the surface. This study investigated structural transformation of biogenic birnessite by accommodating commonly occurring natural heavy metals (Zn, Ni) during the formation of Mn oxides and the influence of those metals on the adsorption behavior of Cs on Mn oxides. It was found that the presence of heavy metals during bio-oxidation of Mn(II), followed by exposure to a low pH aqueous solution, increased the number of available layer vacancies, which consequently increased the adsorption capacity of Cs in the final product birnessite.
Yu, Q.*; Onuki, Toshihiko*; Kozai, Naofumi; Sakamoto, Fuminori; Tanaka, Kazuya; Sasaki, Keiko*
Chemical Geology, 470, p.141 - 151, 2017/10
Times Cited Count:15 Percentile:54.72(Geochemistry & Geophysics)In this work, the Cs retention onto two types of Mn oxide was investigated. We found that Todorokite has sorption sites with a higher selectivity for Cs than birnessite. When the initial Cs concentration was 10 mol/L for the sorption experiments, approximately 34% of the sorbed Cs was residual in the todorokite after the extraction using 1 M NaCl and NH
mol/L for the sorption experiments, approximately 34% of the sorbed Cs was residual in the todorokite after the extraction using 1 M NaCl and NH Cl; this value was much higher than the results for the Cs-sorbed birnessite. These results strongly suggest that todorokite contributes to the fixation of radioactive Cs in soils.
Cl; this value was much higher than the results for the Cs-sorbed birnessite. These results strongly suggest that todorokite contributes to the fixation of radioactive Cs in soils.
 thin films
thin filmsYanagida, Tsuyoshi*; Saito, Yuji; Takeda, Yukiharu; Fujimori, Atsushi*; Tanaka, Hidekazu*; Kawai, Tomoji*
Physical Review B, 79(13), p.132405_1 - 132405_4, 2009/04
Times Cited Count:11 Percentile:45.02(Materials Science, Multidisciplinary)Creating a bipolarity of semiconductors has been a key technology to develop recent advanced semiconductor devices. Although theoretical calculation predicts the presence of ferromagnetic electron-doped manganites with doping tetravalent cations, the ferromagnetic origin in experiments has been controversial due to the lack of direct experimental evidence. Here, we investigate the ferromagnetism in (La,Ce)MnO thin films by measuring the magnetic circular dichroism in soft X-ray absorption (XMCD). The XMCD measurements clarified that the ferromagnetism is not caused by the presence of Mn
thin films by measuring the magnetic circular dichroism in soft X-ray absorption (XMCD). The XMCD measurements clarified that the ferromagnetism is not caused by the presence of Mn but by self-hole doping for manganese.
but by self-hole doping for manganese.
Hotta, Takashi
Progress in Ferromagnetism Research, p.19 - 38, 2006/00
In manganites, the appearance of the ferromagnetic (FM) metallic phase has been usually rationalized by the so-called double-exchange mechanism, based on a strong Hund's rule coupling between mobile 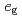 electrons and localized
electrons and localized 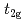 spins. Then, it has been believed for a long time that hole-doping is indispensable for explaining the FM metallic phase in manganites. However, it has been theoretically pointed out that a novel metal-insulator transition in the FM state can occur even for undoped manganites based on the
spins. Then, it has been believed for a long time that hole-doping is indispensable for explaining the FM metallic phase in manganites. However, it has been theoretically pointed out that a novel metal-insulator transition in the FM state can occur even for undoped manganites based on the 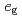 -orbital degenerate Hubbard model tightly coupled to Jahn-Teller distortions. In this article, the result is reviewed in detail.
-orbital degenerate Hubbard model tightly coupled to Jahn-Teller distortions. In this article, the result is reviewed in detail.
 Sr
Sr MnO
MnO studied by resonant inelastic X-ray scattering
studied by resonant inelastic X-ray scatteringIshii, Kenji; Inami, Toshiya; Owada, Kenji; Kuzushita, Kaori; Mizuki, Junichiro; Murakami, Yoichi; Ishihara, Sumio*; Endo, Yasuo*; Maekawa, Sadamichi*; Hirota, Kazuma*; et al.
Journal of Physics and Chemistry of Solids, 66(12), p.2157 - 2162, 2005/12
Times Cited Count:0 Percentile:0(Chemistry, Multidisciplinary)We have studied electronic excitations of hole-doped manganese oxides La Sr
Sr MnO
MnO (
(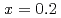 and
and 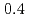 ) by resonant inelastic X-ray scattering at Mn K absorption edge. We observed in the excitation spectra that the Mott gap of La
) by resonant inelastic X-ray scattering at Mn K absorption edge. We observed in the excitation spectra that the Mott gap of La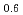 Sr
Sr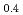 MnO
MnO was filled by hole-doping. In La
was filled by hole-doping. In La Sr
Sr MnO
MnO , which shows a metal-insulator transition, we found that temperature dependence of scattering intensity is different between two scattering vectors. It may reflects a anisotropic electronic structure which originates from the orbital degrees of freedom in manganese oxides.
, which shows a metal-insulator transition, we found that temperature dependence of scattering intensity is different between two scattering vectors. It may reflects a anisotropic electronic structure which originates from the orbital degrees of freedom in manganese oxides.
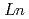
 Ca
Ca MnO
MnO (
(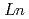 =Ho, Er, Tm, Yb and Lu)
=Ho, Er, Tm, Yb and Lu)Yoshii, Kenji; Abe, Hideki*; Ikeda, Naoshi*
Journal of Solid State Chemistry, 178(12), p.3615 - 3623, 2005/12
Times Cited Count:25 Percentile:67.27(Chemistry, Inorganic & Nuclear)no abstracts in English
 Sr
Sr MnO
MnO (
(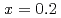 ,
, 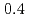 )
)Ishii, Kenji; Inami, Toshiya; Owada, Kenji; Kuzushita, Kaori; Mizuki, Junichiro; Murakami, Yoichi; Ishihara, Sumio*; Endo, Yasuo*; Maekawa, Sadamichi*; Hirota, Kazuma*; et al.
Physical Review B, 70(22), p.224437_1 - 224437_6, 2004/12
Times Cited Count:19 Percentile:64.23(Materials Science, Multidisciplinary)Electronic excitations in the hole doped manganese oxides (La Sr
Sr MnO
MnO ,
, 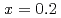 and
and 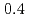 ) have been elucidated by using the resonant inelastic X-ray scattering (RIXS) method. A doping effect in the strongly correlated electron systems has been observed for the first time. The scattering spectra show that a salient peak appears in low energies indicating the persistence of the Mott gap. At the same time, the energy gap is partly filled by doping holes and the spectral weight energy shifts toward lower energies. The excitation spectra show little change in the momentum space as is in undoped LaMnO
) have been elucidated by using the resonant inelastic X-ray scattering (RIXS) method. A doping effect in the strongly correlated electron systems has been observed for the first time. The scattering spectra show that a salient peak appears in low energies indicating the persistence of the Mott gap. At the same time, the energy gap is partly filled by doping holes and the spectral weight energy shifts toward lower energies. The excitation spectra show little change in the momentum space as is in undoped LaMnO . On the other hand, the scattering intensities in the low energy excitations of
. On the other hand, the scattering intensities in the low energy excitations of 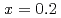 are anisotropic in temperature dependence, which indicates an anisotropy of magnetic interaction and underlying effect of the orbital.
are anisotropic in temperature dependence, which indicates an anisotropy of magnetic interaction and underlying effect of the orbital.
Takahashi, Manabu*; Tanaka, Kazuya*; Tamada, Masao; Aoi, Toru*
Kankyo Kogaku Kenkyu Rombunshu, Vol.41, p.229 - 235, 2004/11
Fibrous metal adsorbent having iminodiacetic acid was synthesized by radiation-induced grafting glycidyl methacrylate on nonwoven fabric and subsequent chemical treatment. The degree of grafting calculated by increasing weight after grafting reached 170 % for reaction time of 2h at 40  C. The adsorption characteristics of ferric and manganese ions were evaluated by using the resulting adsorbent with 2.1 mmol/g-adsorbent function group of iminodiacetic acid. Each distribution coefficient of ferric and manganese ion deceased with increase of another coexist ion. Both ferric and manganese ions were completely removed by the adsorbent column at the space velocity of 1000h
C. The adsorption characteristics of ferric and manganese ions were evaluated by using the resulting adsorbent with 2.1 mmol/g-adsorbent function group of iminodiacetic acid. Each distribution coefficient of ferric and manganese ion deceased with increase of another coexist ion. Both ferric and manganese ions were completely removed by the adsorbent column at the space velocity of 1000h . Adsorption capacities of both ions were reduced to 80% after 5 times reputation of adsorption and desorption.
. Adsorption capacities of both ions were reduced to 80% after 5 times reputation of adsorption and desorption.
Otosaka, Shigeyoshi; Togawa, Orihiko; Baba, Masami*; Karasev, E.*; Volkov, Y. N.*; Omata, Nobutaka*; Noriki, Shinichiro*
Marine Chemistry, 91(1-4), p.143 - 163, 2004/11
Times Cited Count:42 Percentile:71.92(Chemistry, Multidisciplinary)Spatial and temporal variations of particulate flux were observed by sediment trap experiments at three areas of the Japan Sea (western Japan Basin, eastern Japan Basin and Yamato Basin) during 1999-2002. Mass flux in the Japan Sea showed remarkable regional distribution. Annual mean mass flux at 1 km depth was 455 mg/m /day in the eastern Japan Basin, 252 mg/m
/day in the eastern Japan Basin, 252 mg/m /day in the eastern Japan Basin and 147 mg/m
/day in the eastern Japan Basin and 147 mg/m /day in the Yamato Basin. Mass fluxes were especially large in spring (March-May). From the distribution of elemental abundance in sediments, La/Yb and Mn/Al ratios as indicators of the origin of aluminosilicates and the "freshness" of particles, respectively. These proxies suggested three sources of lithogenic material for the Japan Sea, (1) atmospheric input of Kosa particles, (2) lateral transport from the East China Sea, and (3) lateral transport from Island-Arc such as the Japan Islands.
/day in the Yamato Basin. Mass fluxes were especially large in spring (March-May). From the distribution of elemental abundance in sediments, La/Yb and Mn/Al ratios as indicators of the origin of aluminosilicates and the "freshness" of particles, respectively. These proxies suggested three sources of lithogenic material for the Japan Sea, (1) atmospheric input of Kosa particles, (2) lateral transport from the East China Sea, and (3) lateral transport from Island-Arc such as the Japan Islands.
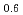 Ru
Ru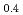 O
O
Yoshii, Kenji; Nakamura, Akio; Mizumaki, Masaichiro*; Tanida, Hajime*; Kawamura, Naomi*; Abe, Hideki*; Ishii, Yoshinobu; Shimojo, Yutaka; Morii, Yukio
Journal of Magnetism and Magnetic Materials, 272-276(Suppl.), p.e609 - e611, 2004/05
Times Cited Count:4 Percentile:25.08(Materials Science, Multidisciplinary)no abstracts in English